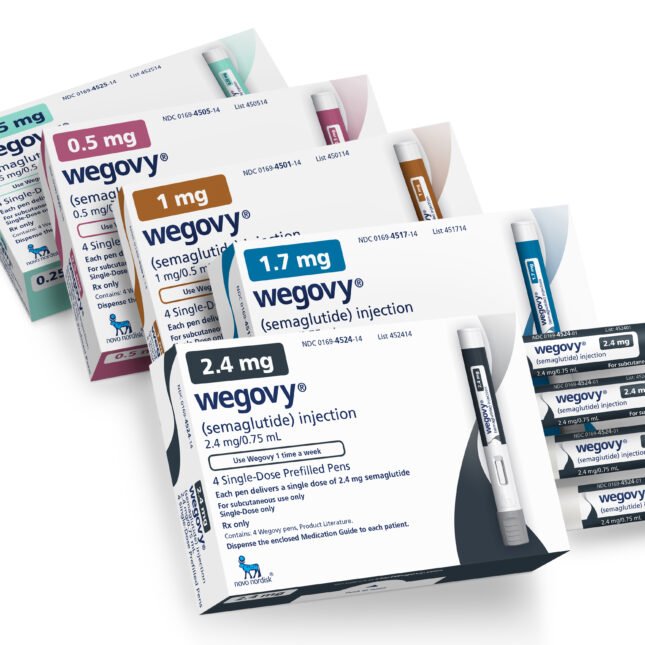
- Wegovy
- semaglutide
- semaglutide
- Obesity
- Overweight
- A10BJ06
Pharmacotherapeutic group
Antiobesity preparations, excl. diet products
Therapeutic indication
Wegovy is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of
– ?30 kg/m² (obesity), or
– ?27 kg/m² to <30 kg/m² (overweight) in the presence of at least one weight-related comorbidity e.g. dysglycaemia (prediabetes or type 2 diabetes mellitus), hypertension, dyslipidaemia, obstructive sleep apnoea or cardiovascular disease.
Adolescents (?12 years)
Wegovy is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adolescents ages 12 years and above with
• obesity* and
• body weight above 60 kg.
Treatment with Wegovy should be discontinued and re-evaluated if adolescent patients have not reduced their BMI by at least 5% after 12 weeks on the 2.4 mg or maximum tolerated dose.
*Obesity (BMI ?95th percentile) as defined on sex- and age-specific BMI growth charts (CDC.gov) (see Table 1 in 4.1 of SmPC).
Table 1 BMI cut-off points for obesity (?95th percentile) by sex and age for paediatric patients aged 12 and older (CDC criteria)



Reviews
There are no reviews yet.